'Xiao Yao' - Sodium ion battery
1、 Preface
On the afternoon of October 24th, 2024, the Ningde Times Super Hybrid Battery Brand and New Product Launch Conference was held.

Since the birth of hybrid vehicles, CATL has attached great importance to the research and development of hybrid batteries. The relevant person in charge of CATL stated, "In recent years, we have continuously increased our research and development investment, collaborated with host manufacturers and partners, and launched generation after generation of new technologies and products.
From the current market situation, the development of hybrid models also faces some problems: firstly, the pure electric range is short, generally only able to run in the city, requiring frequent charging; The second reason is weak low-temperature performance, and the battery life decreases rapidly in winter, basically requiring a "fold in half"; The third issue is slow charging speed. Due to the fact that hybrid models generally do not have fast charging, although the battery is small, the charging speed is not much different from that of cars with pure large batteries.
In order to solve the above problems, CATL has released the "Xiao Yao" super hybrid battery, which is the world's first pure electric hybrid battery with a range of over 400 kilometers and 4C supercharging function. It can last 280 kilometers in 10 minutes of charging and can run for a week, achieving the goal of "charging for one moment, Xiao Yao for seven days".
The "Xiao Yao" super hybrid battery has achieved the commercial application of sodium ion battery technology, which can discharge the battery in extremely cold environments of minus 40 degrees Celsius, charge it at minus 30 degrees Celsius, and provide strong power output at minus 20 degrees Celsius, which is basically no different from the normal temperature state.
2、 So what is a sodium ion battery?
Sodium ion battery is a type of secondary battery that relies on the movement of sodium ions between the positive and negative electrodes for charging and discharging. Its working principle and manufacturing process are similar to lithium batteries, and both belong to the rocking chair type battery. It is mainly composed of positive and negative electrode materials, electrolyte, separator, and shell. Compared with lithium batteries, sodium battery products have the advantages of strong resource availability, comprehensive cost advantage in scale, excellent rate performance, and high low-temperature capacity retention rate.
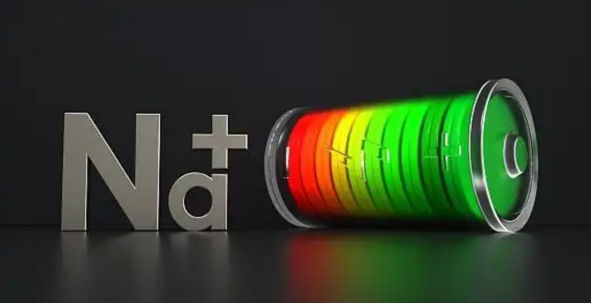
The following table compares the characteristics of sodium ion batteries using conventional lithium iron phosphate batteries as templates.
Principle and reaction equation of sodium ion battery: Sodium ion battery is essentially the same as lithium-ion battery. When not initially charged or discharged, the positive electrode is a sodium rich material and the negative electrode is a sodium poor material; During charging, sodium ions are released from the positive electrode and embedded in the negative electrode through the electrolyte and separator. Electrons are compensated to the negative electrode through an external circuit to ensure charge balance between the positive and negative electrodes; When discharging, it is the opposite.

3.Classification of Sodium Ion Batteries
(1) Classify according to the positive electrode material system
According to the crystal structure of the positive electrode material system, sodium ion batteries can be divided into oxide (including layered and tunnel structures), Prussian blue, fluorinated phosphate, phosphate, sulfate, and organic compound sodium ion batteries. At present, there are three main technological routes that have been extensively studied: layered transition metal oxides, polyanions, and Prussian blue. Similar to the positive electrode materials of lithium-ion batteries, most sodium ion battery positive electrode materials also include variable valence transition metal elements. Different transition metal elements exhibit different oxidation-reduction potentials and electron numbers in different crystal structures.
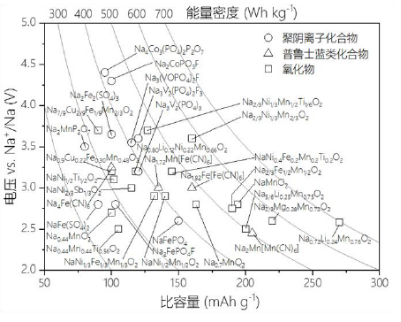
The above figure summarizes the basic electrochemical parameters of the three common positive electrode materials for sodium ion batteries. Layered oxides have periodic layered structures, simple preparation methods, high capacity and voltage, and are the main positive electrode materials for sodium ion batteries. Due to their high specific capacity and energy density, as well as low production costs, they are expected to become mainstream sodium ion battery systems with good application prospects. In addition, the energy density of such materials can be increased through the reaction of lattice oxygen. However, layered materials are mostly prone to absorbing water or reacting with air, which affects the stability and electrochemical performance of the structure and cannot be stored in air for a long time. Polyanion positive electrode materials mostly have an open three-dimensional skeleton, good rate performance, and cycling performance. Among them, fluorophosphate has a high oxidation-reduction potential, but fluorine may corrode equipment and have an impact on the environment during manufacturing and recycling processes. For polyanionic compounds, their conductivity is generally poor, and in order to improve their electronic conductivity, it is often necessary to adopt methods such as nanomaterialization combined with carbon coating, which will result in a low volumetric energy density. Prussian blue is a new type of cathode material with great potential that has been developed in recent years. It has an open three-dimensional channel (framework structure) that allows Na+to migrate rapidly in the tunnel, thus exhibiting good structural stability and high current output performance. However, many problems still need to be solved before the practical application of Prussian blue compounds, such as difficulty in removing crystal water and dissolution of transition metals. Organic positive electrode materials generally have high specific capacity due to their multi electron reaction characteristics, and can reversibly achieve Na+extraction and insertion; However, their kinetics are relatively slow, their electronic conductivity is mostly poor, and they may also dissolve in the electrolyte. The performance of such materials needs further improvement. In addition to the above materials, conversion materials such as transition metal selenides, halides, and sulfides can achieve multi electron transfer reactions and generally have high capacities; However, the drawbacks of such materials are also evident, such as low electronic conductivity, slow kinetics, large volume changes, severe voltage hysteresis, and low operating voltage, which limit their practical applications.
(2)Classify according to the negative electrode material system
Negative electrode materials are the main sodium ion storage carriers during the charging process. According to their storage characteristics, sodium ion batteries can be divided into carbon based, embedded titanium based, organic based, alloy based, and conversion based sodium ion batteries, as shown in the following figure.
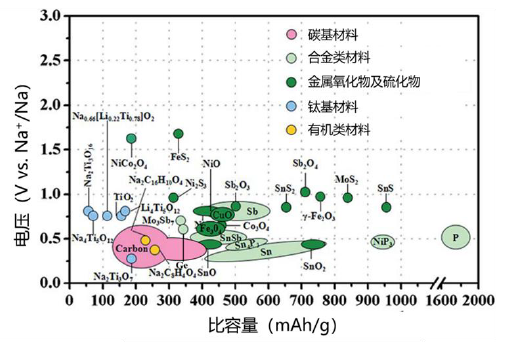
Carbon based is currently the most promising negative electrode material system for sodium ion batteries. At present, carbon based materials that can be used as negative electrode materials for batteries mainly include graphite, amorphous carbon, and carbon nanotubes. However, due to thermodynamic reasons, sodium ions cannot be embedded between graphite layers to form stable compounds with carbon, making it difficult for sodium ion batteries to use graphite as a negative electrode material. Carbon nanomaterials mainly include graphene, carbon nanotubes, etc., which rely on surface adsorption to store sodium and achieve rapid charging and discharging. However, there are problems such as very low Coulombic efficiency and poor cycling, making it difficult to obtain practical applications. Amorphous carbon materials with large interlayer spacing have become the most promising negative electrode materials for sodium ion batteries due to their high sodium storage capacity, low sodium storage potential, and excellent cycling stability. They mainly include amorphous carbon materials such as soft carbon, hard carbon, and soft hard composite carbon.
Embedded titanium based materials have good stability in air, and the oxidation-reduction potential of Ti4+/Ti3+is between 0V~2V (vs. Na+/Na). Different structures exhibit different sodium storage potentials. As an important research object for negative electrode materials in sodium ion batteries, titanium based materials have received widespread attention.
Organic compounds have rich chemical compositions, wide sources of raw materials, low costs, environmental friendliness, and adjustable electrochemical windows and multi electron reactions, which have aroused great interest among researchers as negative electrode materials for sodium ion batteries. However, the biggest problem with organic compounds is that their electronic conductivity is relatively poor and they are easily soluble in electrolytes. How to improve the electronic conductivity of materials is the key to solving the practical application of organic compounds. Although carboxylic acid organic molecules can provide relatively good electrochemical performance in terms of capacity and operating voltage, their rate performance and cycling performance still need to be improved. The research focus of this type of material is to improve the performance of organic negative electrode materials for sodium ion batteries by adjusting their molecular structure, surface coating, polymerization, and other methods.
Na-M (M=Si, Ge, Sn, Pb, P, As, Sb, Bi) alloy materials, as negative electrode materials for sodium ion batteries, have high theoretical capacity, low sodium storage potential, good conductivity, and can also avoid dendrite problems caused by sodium elemental substances, thereby improving their safety. The emergence of sodium alloys has to some extent solved the potential safety hazards of metal sodium negative electrodes. However, sodium alloys undergo significant volume changes during repeated cycling, causing electrode materials to gradually pulverize and battery capacity to rapidly decline. Therefore, for alloy materials, improving their cycling stability is the focus of research. There is still a long way to go for sodium alloys to be used as negative electrode materials for sodium secondary batteries.
Other materials include metal oxides (such as Fe2O3, CuO, CoO, MoO3, NiCo2O4, etc.) and sulfides (such as MoS2, SnS, etc.). Metal oxide negative electrode materials have poor conductivity, and are prone to aggregation and irreversible conversion reactions. During cycling, they undergo significant volume expansion, which damages the integrity of the electrode material and leads to poor cycling stability and rate performance. Therefore, it is necessary to design some new metal oxides with micro nano structures to improve the electrochemical performance of materials.
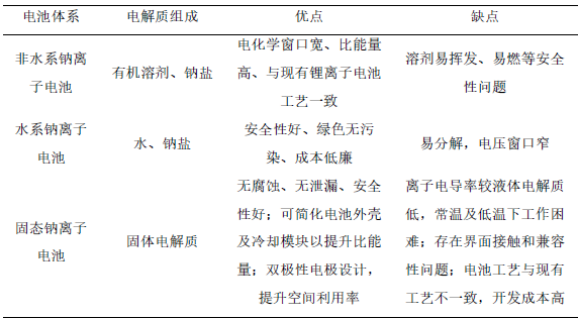
(3)Classified by electrolyte system
According to the type of electrolyte, sodium ion batteries can be classified into solid-state sodium ion batteries and liquid sodium ion batteries, among which liquid sodium ion batteries include aqueous sodium ion batteries and non-aqueous sodium ion batteries. The detailed comparison is shown in the table below.
Sodium ion batteries can be classified not only according to their material system, but also based on the packaging form used. Specifically, in terms of battery structure, they can be mainly divided into three categories: cylindrical, flexible packaging, and square hard shell. If the specific process is not considered, these three categories are basically the same as lithium-ion batteries.
4、 Characteristics of Sodium Ion Batteries
(1) High and low temperature characteristics
Compared with lithium-ion batteries, sodium ion batteries have much better low-temperature performance. The ion radius of sodium ion batteries is larger than that of lithium-ion batteries, so the desolvation energy of sodium ions is relatively low. The desolvation process is also faster at low temperatures, and the concentration of salt in the sodium ion electrolyte solution will be lower. At low temperatures, the ion conductivity will be higher and the viscosity will be lower.
Secondly, the electrolyte of sodium ion batteries usually uses inorganic salts or aqueous solutions. These electrolytes have higher ion conductivity and lower freezing temperature, effectively preventing electrolyte freezing and electrode damage, thereby ensuring the low-temperature performance of sodium ion batteries.
Sodium ions have high ion conductivity, require lower electrolyte concentration, and have lower electrolyte viscosity than lithium-ion batteries at low temperatures. Compared to the working temperature range of -20 ℃ to 60 ℃ for lithium-ion batteries, sodium ion batteries can operate normally in the temperature range of -40 ℃ to 80 ℃.
(2)Safety features
Compared to other batteries, sodium ion batteries have higher stability and are less prone to thermal runaway and other issues. Lithium ion batteries are prone to dendrite formation at high current densities, which may puncture the internal structure of the battery, leading to short circuits and spontaneous combustion. The probability of dendrite formation in sodium ion batteries is relatively low, so they are unlikely to encounter such safety issues. In addition, sodium ion batteries can operate normally over a wide temperature range (from -40 ° C to 80 ° C), and even in an environment of -20 ° C, their capacity retention rate is close to 90%, indicating that their high and low temperature performance is superior to other secondary batteries.
Sodium ion batteries have higher thermal runaway temperatures and are prone to non self ignition due to passivation and oxidation in high-temperature environments. The electrochemical window of sodium salt electrolyte is larger, the possibility of electrolyte decomposition during the reaction process is lower, and the stability of the battery system is higher. Sodium ion battery chemistry allows the use of aluminum metal as a current collector at the anode, which can effectively avoid the over discharge problem of graphite based lithium-ion batteries. Moreover, the internal resistance of sodium ion batteries is higher than that of lithium batteries, so they generate less instantaneous heat in the event of a short circuit, have lower temperature rise, and have a higher thermal runaway temperature than lithium batteries, providing higher safety.
Note: The data and images cited in the article are sourced from the internet